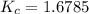Chemistry, 30.03.2020 19:34 jallison61
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially dissociates by the reaction below. 2 NH3(g) equilibrium reaction arrow N2(g) 3 H2(g) At equilibrium, 2.0 mol NH3 remains. What is the value of K for this reaction

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 22:30
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
At a certain temperature, 4.0 mol NH3 is introduced into a 2.0 L container, and the NH3 partially di...
Questions




French, 10.11.2020 05:10



History, 10.11.2020 05:10


Mathematics, 10.11.2020 05:10

Mathematics, 10.11.2020 05:10

History, 10.11.2020 05:10

Biology, 10.11.2020 05:10



Mathematics, 10.11.2020 05:10



Mathematics, 10.11.2020 05:10


Mathematics, 10.11.2020 05:10

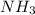 = 4.0 mole
= 4.0 mole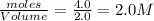
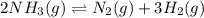
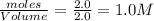
![K_c=\frac{[x]\times [3x]^3}{[(2-2x)]^2}](/tpl/images/0570/9112/70e97.png)
![K_c=\frac{[0.5]\times [3\times 0.5]^3}{[(2-2\times 0.5)]^2}](/tpl/images/0570/9112/97489.png)