
Chemistry, 30.03.2020 20:20 preciadogabriel40
Find the pH during the titration of 20.00 mL of 0.1000 M butanoic acid, CH3CH2CH2COOH (K a = 1.54 × 10 − 5), with 0.1000 M NaOH solution after the following additions of titrant (total volume of added base given):

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2
You know the right answer?
Find the pH during the titration of 20.00 mL of 0.1000 M butanoic acid, CH3CH2CH2COOH (K a = 1.54 ×...
Questions



Mathematics, 25.10.2021 06:30



Mathematics, 25.10.2021 06:30

Mathematics, 25.10.2021 06:30

English, 25.10.2021 06:30

Biology, 25.10.2021 06:30

Mathematics, 25.10.2021 06:30




Geography, 25.10.2021 06:30


Arts, 25.10.2021 06:30

Mathematics, 25.10.2021 06:30

Mathematics, 25.10.2021 06:40


Chemistry, 25.10.2021 06:40

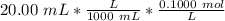
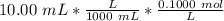
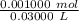
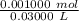
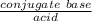
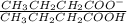
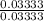
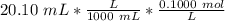
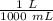
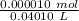

![[H_3O^+] = \frac{K \omega }{[OH^-]}](/tpl/images/0570/9707/cb9be.png)
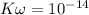
![[H_3O^+] = \frac{1.0*10^{-14}}{2.494*10^{-14}}](/tpl/images/0570/9707/791bd.png)
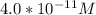
![[H_3O^+}]](/tpl/images/0570/9707/f6691.png)
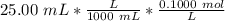
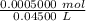
![[H_3O^+] = \frac{K \omega }{[OH^+]}](/tpl/images/0570/9707/32cc9.png)
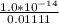
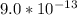 M
M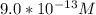 ]
]

