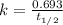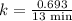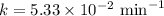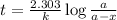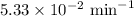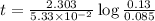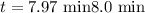Chemistry, 30.03.2020 20:27 smancilla2020
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. A) 12 B) 10. C) 8.0 D) 11 E) 5.0 C

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13...
Questions


Social Studies, 28.09.2019 05:20



Physics, 28.09.2019 05:20

Chemistry, 28.09.2019 05:20


Mathematics, 28.09.2019 05:20

Spanish, 28.09.2019 05:20



Biology, 28.09.2019 05:20

History, 28.09.2019 05:20

Biology, 28.09.2019 05:20


Arts, 28.09.2019 05:20

Mathematics, 28.09.2019 05:20



Social Studies, 28.09.2019 05:20