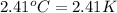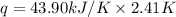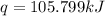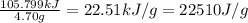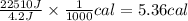A researcher studying the nutritional value of a new candy places a 4.70 g 4.70 g sample of the candy inside a bomb calorimeter and combusts it in excess oxygen. The observed temperature increase is 2.41 ∘ C. 2.41 ∘C. If the heat capacity of the calorimeter is 43.90 kJ ⋅ K − 1 , 43.90 kJ⋅K−1, how many nutritional Calories are there per gram of the candy?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2
You know the right answer?
A researcher studying the nutritional value of a new candy places a 4.70 g 4.70 g sample of the cand...
Questions


Geography, 12.03.2021 18:50



Social Studies, 12.03.2021 18:50

Biology, 12.03.2021 18:50




Mathematics, 12.03.2021 18:50


Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50


Social Studies, 12.03.2021 18:50

Chemistry, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

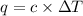
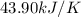
 = change in temperature =
= change in temperature =