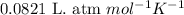Chemistry, 30.03.2020 20:35 esanchez2002fcb
Dry ice is solid carbon dioxide. A 1.65−g sample of dry ice is placed in an evacuated 3.93−L vessel at 23.0°C. Calculate the pressure inside the vessel after all the dry ice has been converted to CO2 gas.

Answers: 3
Another question on Chemistry


Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
Dry ice is solid carbon dioxide. A 1.65−g sample of dry ice is placed in an evacuated 3.93−L vessel...
Questions


English, 06.05.2021 02:20

Mathematics, 06.05.2021 02:20

Mathematics, 06.05.2021 02:20





English, 06.05.2021 02:20

Biology, 06.05.2021 02:20

Mathematics, 06.05.2021 02:20

History, 06.05.2021 02:20

Mathematics, 06.05.2021 02:20

English, 06.05.2021 02:20


Mathematics, 06.05.2021 02:20

Mathematics, 06.05.2021 02:20

Mathematics, 06.05.2021 02:20


Mathematics, 06.05.2021 02:20

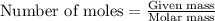
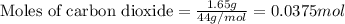
![23^oC=[23+273]=296K](/tpl/images/0571/0457/eb892.png)