

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
Assuming no change in temperature and pressure, calculate the volume of O2 (in liters) required for...
Questions

English, 06.05.2020 07:40

Mathematics, 06.05.2020 07:40





English, 06.05.2020 07:40


Mathematics, 06.05.2020 07:41



Biology, 06.05.2020 07:41

Mathematics, 06.05.2020 07:41

Mathematics, 06.05.2020 07:41

Mathematics, 06.05.2020 07:41


Mathematics, 06.05.2020 07:41



Mathematics, 06.05.2020 07:41

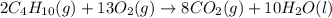
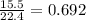 mole of butane gas
mole of butane gas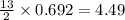 moles of oxygen gas
moles of oxygen gas

