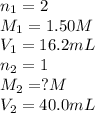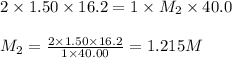Chemistry, 30.03.2020 21:17 nannagarvey9945
A volume of 40.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard solution of sulfuric acid (H2SO4H2SO4). What was the molarity of the KOHKOH solution if 16.2 mLmL of 1.50 MM H2SO4H2SO4 was needed? The equation is 2KOH(aq)+H2SO4(aq)→K2SO4(aq)+2H2O(l )

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

You know the right answer?
A volume of 40.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard soluti...
Questions

Mathematics, 20.04.2022 21:40

Computers and Technology, 20.04.2022 22:00

Mathematics, 20.04.2022 22:00



Mathematics, 20.04.2022 22:40




Mathematics, 20.04.2022 23:20



English, 21.04.2022 01:00


Mathematics, 21.04.2022 01:00

Law, 21.04.2022 01:00



Chemistry, 21.04.2022 01:50

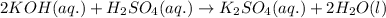
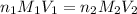
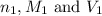 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 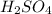
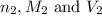 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.