
Chemistry, 30.03.2020 20:46 joannegrace869
Problem PageQuestion Acetylene gas is often used in welding torches because of the very high heat produced when it reacts with oxygen gas, producing carbon dioxide gas and water vapor. Calculate the moles of acetylene needed to produce 2.00 mol of water. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1

Chemistry, 23.06.2019 15:00
Does the formation of all chemical bonds is based on sharing of electrons?
Answers: 1

Chemistry, 23.06.2019 17:30
The are found on the right side of the arrow in a chemical reaction. a. reactants b. products c. subscripts d. coefficients reset
Answers: 1
You know the right answer?
Problem PageQuestion Acetylene gas is often used in welding torches because of the very high heat pr...
Questions

History, 19.03.2021 14:00



Social Studies, 19.03.2021 14:00




Mathematics, 19.03.2021 14:00

English, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00


Chemistry, 19.03.2021 14:00



English, 19.03.2021 14:00


Mathematics, 19.03.2021 14:00

Mathematics, 19.03.2021 14:00

Physics, 19.03.2021 14:00

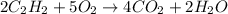
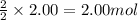 of acetylene gas
of acetylene gas

