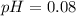Chemistry, 30.03.2020 21:10 Franklyn3220
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enough water to make a final volume of 75.0 mLmL. Part A Assuming that all of the solid dissolves, what is the pH of the final solution

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enoug...
Questions




Mathematics, 10.06.2021 21:30



Engineering, 10.06.2021 21:30





Mathematics, 10.06.2021 21:30

English, 10.06.2021 21:30

Biology, 10.06.2021 21:30

Mathematics, 10.06.2021 21:30



Mathematics, 10.06.2021 21:30

Mathematics, 10.06.2021 21:30

Biology, 10.06.2021 21:30

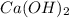 =
=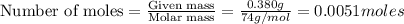
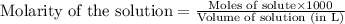
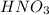 solution = 1.45 M
solution = 1.45 M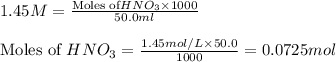
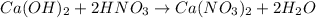
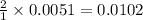 moles of
moles of 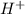 are left in 75.0 ml of solution
are left in 75.0 ml of solution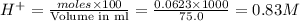
![pH=-\log [H^+]](/tpl/images/0571/2204/37e81.png)
![pH=-\log[0.83]](/tpl/images/0571/2204/f1115.png)