
Chemistry, 30.03.2020 21:03 kharmaculpepper
When coal is burned, the sulfur present in coal is converted to sulfur dioxide (SO2), which is responsible for the acid rain phenomenon. S(s) + O2(g) → SO2(g) If 4.10 kg of S are reacted with oxygen, calculate the volume of SO2 gas (in mL) formed at 30°C and 1.16 atm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1
You know the right answer?
When coal is burned, the sulfur present in coal is converted to sulfur dioxide (SO2), which is respo...
Questions

Spanish, 13.08.2021 04:00



Mathematics, 13.08.2021 04:00

Mathematics, 13.08.2021 04:00


Social Studies, 13.08.2021 04:00






Social Studies, 13.08.2021 04:10

Mathematics, 13.08.2021 04:10

Mathematics, 13.08.2021 04:10

English, 13.08.2021 04:10


Mathematics, 13.08.2021 04:10

Law, 13.08.2021 04:10

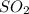 gas is,
gas is, 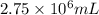
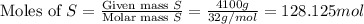
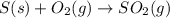
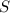 react to give 1 mole of
react to give 1 mole of 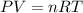
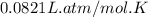
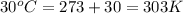
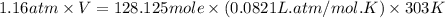
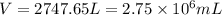 (1 L = 1000 mL)
(1 L = 1000 mL)

