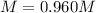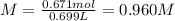Chemistry, 30.03.2020 21:05 jailenevazquez755
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 mL of water to form hydrochloric acid solution. Calculate the molarity of the solution. Assume no change in volume.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 m...
Questions

SAT, 15.01.2021 17:50




History, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50

Biology, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50



Mathematics, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50


Mathematics, 15.01.2021 17:50

Chemistry, 15.01.2021 17:50


Mathematics, 15.01.2021 17:50


Mathematics, 15.01.2021 17:50

Mathematics, 15.01.2021 17:50