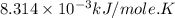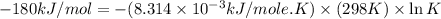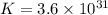Chemistry, 30.03.2020 21:03 manyah6189
Silver occurs in trace amounts in some ores of lead, and lead can displace silver from solution: Pb(s) + 2Ag+ (aq) LaTeX: \longrightarrow⟶ Pb2+(aq) + 2Ag(s) As a consequence, silver is a valuable byproduct in the industrial extraction of lead from its ores. Calculate K and LaTeX: \DeltaΔG° at 298K for this reaction. E°cell = .93 K: Enter as e notation to 1 decimal place (eg 1.2e3) LaTeX: \DeltaΔG°: Enter in kJ/mol to 0 decimal places. Use 96.5 kJ/Vmol e- for F (Faraday's constant). Do not use e notation.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Silver occurs in trace amounts in some ores of lead, and lead can displace silver from solution: Pb(...
Questions










Engineering, 26.07.2019 18:30



Mathematics, 26.07.2019 18:30







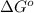 and K is, -180 kJ/mol and
and K is, -180 kJ/mol and 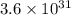
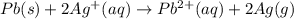
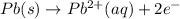
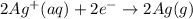
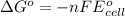
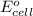 = standard electrode potential of the cell = 0.93 V
= standard electrode potential of the cell = 0.93 V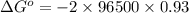
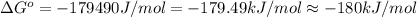
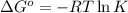
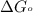 = standard Gibbs free energy = -180 kJ/mol
= standard Gibbs free energy = -180 kJ/mol