
Chemistry, 30.03.2020 21:01 gungamer720
G Identify limiting reactants (mole ratio method). Close Problem Identify the limiting reactant in the reaction of bromine and chlorine to form BrCl, if 29.7 g of Br2 and 11.2 g of Cl2 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1
You know the right answer?
G Identify limiting reactants (mole ratio method). Close Problem Identify the limiting reactant in t...
Questions

Mathematics, 25.06.2021 09:30




Physics, 25.06.2021 09:30


Mathematics, 25.06.2021 09:30

Mathematics, 25.06.2021 09:30





Mathematics, 25.06.2021 09:30

Mathematics, 25.06.2021 09:30

Computers and Technology, 25.06.2021 09:30

Mathematics, 25.06.2021 09:30

History, 25.06.2021 09:30

Law, 25.06.2021 09:30


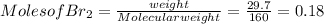
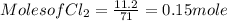
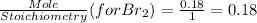
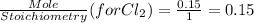
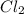 is a limiting reactant and Br₂ is excess reactant.
is a limiting reactant and Br₂ is excess reactant.


