

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3
You know the right answer?
A solution was made by dissolving 1.77 g Ba(OH)2 in 100. mL final volume. (Note: For the purpose of...
Questions

History, 03.12.2021 20:40

Mathematics, 03.12.2021 20:40





Mathematics, 03.12.2021 20:40



Mathematics, 03.12.2021 20:40

Computers and Technology, 03.12.2021 20:40

Biology, 03.12.2021 20:40

English, 03.12.2021 20:40

SAT, 03.12.2021 20:40

Mathematics, 03.12.2021 20:40





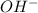 in the solution is 0.2 M
in the solution is 0.2 M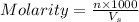
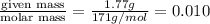
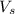 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml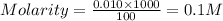
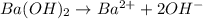
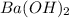 gives 2 moles of
gives 2 moles of 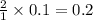 moles of
moles of 

