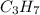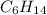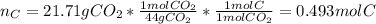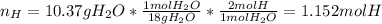Chemistry, 30.03.2020 21:23 jaleelbrown80
When 7.085 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 21.71 grams of CO2 and 10.37 grams of H2O were produced. In a separate experiment, the molar mass of the compound was found to be 86.18 g/mol. Determine the empirical formula and the molecular formula of the hydrocarbon.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
When 7.085 grams of a hydrocarbon, CxHy, were burned in a combustion analysis apparatus, 21.71 grams...
Questions

Chemistry, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

English, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00




Health, 21.09.2021 14:00

History, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

English, 21.09.2021 14:00


Social Studies, 21.09.2021 14:00




Social Studies, 21.09.2021 14:00

History, 21.09.2021 14:00