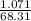A 1.897g sample of Mg(HCO3)2 was heated and decomposed. When the sample
cooled, it weighed 1.0...

Chemistry, 30.03.2020 22:13 FreddyNoTalKing
A 1.897g sample of Mg(HCO3)2 was heated and decomposed. When the sample
cooled, it weighed 1.071g. What is the % yield of this reaction?
Mg(HCO3)2 (s) + CO2 (g) + H2O (g) + MgCO2 (s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3



Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
Questions





Mathematics, 28.07.2021 01:30




Mathematics, 28.07.2021 01:30


Mathematics, 28.07.2021 01:30









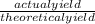 x 100
x 100