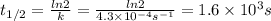Chemistry, 30.03.2020 21:50 karenjjensen
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life for the reaction? 1.9 × 103 s 1.2 × 103 s 1.6 × 103 s 3.0 × 10-4 s

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 23.06.2019 01:30
What is produced from neutralization of an acid and a base? a. hydronium ions b. citric acid c. salt and water
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 09:20
1) a. water molecule breaks up into hydrogen and oxygen on passing electricity. does this involve breaking intermolecular or intramolecular forces of attraction. explain b. on boiling water changes to water vapor. does this involve breaking intermolecular or intramolecular forces of attraction. explain methanol evaporates faster than water. contrast the intermolecular forces and the vapor pressures of methanol and water?
Answers: 2
You know the right answer?
The rate constant, k, for a first-order reaction is equal to 4.3 × 10-4 s-1. What is the half-life f...
Questions

Mathematics, 25.03.2020 20:58


Mathematics, 25.03.2020 20:58




Mathematics, 25.03.2020 20:59

Mathematics, 25.03.2020 20:59



Health, 25.03.2020 20:59

Physics, 25.03.2020 20:59



Mathematics, 25.03.2020 20:59



Social Studies, 25.03.2020 20:59


Mathematics, 25.03.2020 20:59

![rate=k \times [A]^{m}](/tpl/images/0571/4200/f39c7.png)