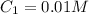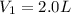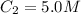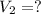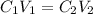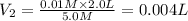The Ksp of barium fluoride, BaF2, is 1.6 x 10-6. A solution of 5.0M NaF is added dropwise to a 2.0L solution that is 0.016 M in Ba2+. When the concentration of fluoride ion exceeds M, BaF2 will precipitate. What volume (in mL) of NaF must be added to cause BaF2 to precipitate? mL

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
The Ksp of barium fluoride, BaF2, is 1.6 x 10-6. A solution of 5.0M NaF is added dropwise to a 2.0L...
Questions





Mathematics, 19.05.2020 03:12

Geography, 19.05.2020 03:12

History, 19.05.2020 03:12

Mathematics, 19.05.2020 03:12



Mathematics, 19.05.2020 03:12

Mathematics, 19.05.2020 03:12


Mathematics, 19.05.2020 03:12

Chemistry, 19.05.2020 03:12




Mathematics, 19.05.2020 03:12

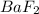 to precipitate.
to precipitate.![[Ba^{2+}]=0.016 M](/tpl/images/0571/5236/69271.png)
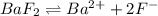
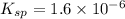
![K_{sp}=[Ba^{2+}][F^-]^2](/tpl/images/0571/5236/2804d.png)
![1.6\times 10^{-6}=[0.016 M]\times [F^-]^2](/tpl/images/0571/5236/5c597.png)
![[F^-]=0.01 M](/tpl/images/0571/5236/09813.png)