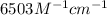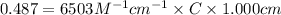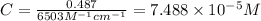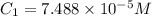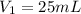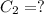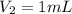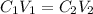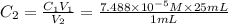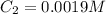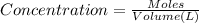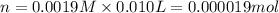An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volumetric flask. A 1.00 mL aliquot of this solution is transferred to a 25 mL volumetric flask, and enough water is added to dilute to the mark. The absorbance of this diluted solution at 327 nm is 0.487 in a 1.000 cm cuvette. The molar absorptivity for this compound at 327 nm is ϵ 327 = 6503 M^(−1) cm^(−1).
(a) What is the concentration of the compound in the cuvette?
(b) What is the concentration of the compound in the 10-mL flask?
(c) How many milligrams of the compound were used to make the 10-mL solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

You know the right answer?
An unknown amount of a compound with a molecular mass of 264.37 g/mol is dissolved in a 10 mL volume...
Questions

Social Studies, 25.06.2019 00:30




Mathematics, 25.06.2019 00:30

Biology, 25.06.2019 00:30

Biology, 25.06.2019 00:30

History, 25.06.2019 00:30

Mathematics, 25.06.2019 00:30

Biology, 25.06.2019 00:30

English, 25.06.2019 00:30

Geography, 25.06.2019 00:30

English, 25.06.2019 00:30

Chemistry, 25.06.2019 00:30


Chemistry, 25.06.2019 00:30

English, 25.06.2019 00:30

Mathematics, 25.06.2019 00:30


Mathematics, 25.06.2019 00:30

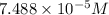 is the concentration of the compound in the cuvette.
is the concentration of the compound in the cuvette.
 = molar absorptivity of this solution =
= molar absorptivity of this solution =