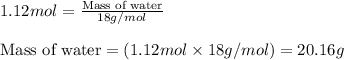Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 13. g of butane is mixed with 70.9 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1
You know the right answer?
Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water ....
Questions

Mathematics, 03.12.2019 06:31

Mathematics, 03.12.2019 06:31


English, 03.12.2019 06:31

History, 03.12.2019 06:31

Mathematics, 03.12.2019 06:31






History, 03.12.2019 06:31

History, 03.12.2019 06:31

Mathematics, 03.12.2019 06:31


Mathematics, 03.12.2019 06:31


History, 03.12.2019 06:31

History, 03.12.2019 06:31

Mathematics, 03.12.2019 06:31

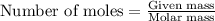 .....(1)
.....(1)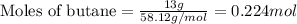
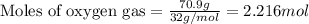
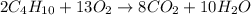
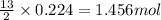 of oxygen gas
of oxygen gas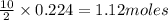 of water
of water