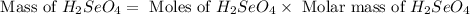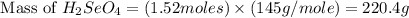Chemistry, 30.03.2020 22:32 savannahvargas512
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O. Calculate the maximum mass (in grams) of selenic acid, H2SeO4, that can be produced in the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Determine the limiting reactant in a mixture containing 139 g of Se, 431 g of Cl2, and 110 g of H2O....
Questions


French, 07.07.2021 21:10

Business, 07.07.2021 21:10



Mathematics, 07.07.2021 21:10



Arts, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10


Mathematics, 07.07.2021 21:10

Mathematics, 07.07.2021 21:10





Mathematics, 07.07.2021 21:10

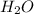
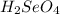 produced is, 220.4 grams.
produced is, 220.4 grams.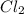 = 431 g
= 431 g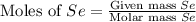
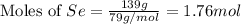
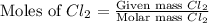
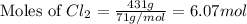
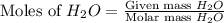
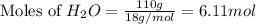
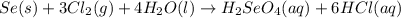
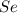
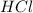 react with
react with 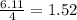 moles of
moles of 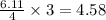 moles of
moles of 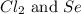 are excess reagent because the given moles are greater than the required moles and
are excess reagent because the given moles are greater than the required moles and