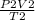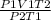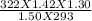Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
A sample of gas initially has a volume of 1.42 L at 293 K and 1.30 atm. What volume will the sample...
Questions



Social Studies, 27.06.2019 02:30

History, 27.06.2019 02:30

Mathematics, 27.06.2019 02:30


Social Studies, 27.06.2019 02:30

Chemistry, 27.06.2019 02:30


Mathematics, 27.06.2019 02:30

Mathematics, 27.06.2019 02:30



English, 27.06.2019 02:30

English, 27.06.2019 02:30

Mathematics, 27.06.2019 02:30

Health, 27.06.2019 02:30


Mathematics, 27.06.2019 02:30

Mathematics, 27.06.2019 02:30

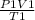 =
=