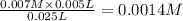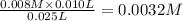A student working in the laboratory prepared the following reactants: 5 mL of 0.007M Cd2+(aq) 10 mL of 0.008M SCN-(aq) 10 mL of 0.5M HNO3(aq) These reagents were mixed and allowed to stand for 10 minutes. The concentration of Cd(SCN)+ in the resulting equilibrium mixture is found to be 5 x 10−4M. Calculate the initial concentration of Cd2+(aq).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
A student working in the laboratory prepared the following reactants: 5 mL of 0.007M Cd2+(aq) 10 mL...
Questions

Mathematics, 02.04.2020 02:43

Mathematics, 02.04.2020 02:43

History, 02.04.2020 02:43

Mathematics, 02.04.2020 02:43



Mathematics, 02.04.2020 02:43

Social Studies, 02.04.2020 02:43




Mathematics, 02.04.2020 02:43

English, 02.04.2020 02:43


Mathematics, 02.04.2020 02:43

Mathematics, 02.04.2020 02:43

Biology, 02.04.2020 02:43


Mathematics, 02.04.2020 02:43

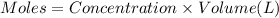
![[Cd^{2+}]=0.007M](/tpl/images/0571/8149/5e85a.png)
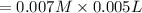
![[SCN^{-}]=0.008 M](/tpl/images/0571/8149/33083.png)
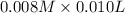
![[HNO_3]=0.5 M](/tpl/images/0571/8149/6e078.png)