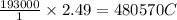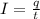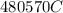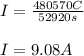Chemistry, 30.03.2020 23:09 ezrabridger49
A 1.0 L solution of CaF2 was electrolyzed for 14.7 h to give 99.47 g of calcium. Assuming the minimum voltage needed was avalible, what amperage would be needed to complete the electrolysis in the given time

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1
You know the right answer?
A 1.0 L solution of CaF2 was electrolyzed for 14.7 h to give 99.47 g of calcium. Assuming the minimu...
Questions


History, 29.09.2021 22:10

History, 29.09.2021 22:10


History, 29.09.2021 22:10

Mathematics, 29.09.2021 22:10



Social Studies, 29.09.2021 22:10

Mathematics, 29.09.2021 22:10

Mathematics, 29.09.2021 22:10


Mathematics, 29.09.2021 22:10

Physics, 29.09.2021 22:10


English, 29.09.2021 22:10

Social Studies, 29.09.2021 22:10

Biology, 29.09.2021 22:10

Arts, 29.09.2021 22:10

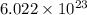 number of particles.
number of particles.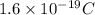
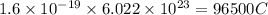
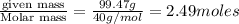
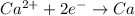
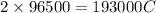 is required to deposit = 1 mole
is required to deposit = 1 mole