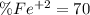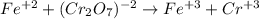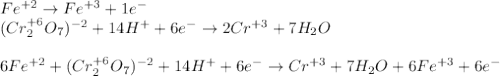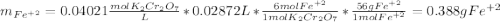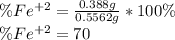Chemistry, 30.03.2020 23:08 GreenHerbz206
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solution. This solution is then titrated with 28.72 mL of 0.04021 M K2Cr2O7 (aq). What is the percent by mass iron in the ore sample

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
An iron ore sample weighing 0.5562 g is dissolved HCl (aq), and the iron is obtained as Fe2 in solut...
Questions

Computers and Technology, 31.12.2021 14:00


Mathematics, 31.12.2021 14:00

Health, 31.12.2021 14:00


History, 31.12.2021 14:00

History, 31.12.2021 14:00



Business, 31.12.2021 14:00

History, 31.12.2021 14:00


Chemistry, 31.12.2021 14:00






History, 31.12.2021 14:00

Computers and Technology, 31.12.2021 14:00