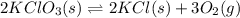Chemistry, 30.03.2020 22:56 prince2195
Write an equilibrium expression for each chemical equation involving one or more solid or liquid reactants or products. a. CO3 2-(aq) + H2O(I)HCO3 -(aq) + OH-(aq) b. 2 KCIO3(s)2 KCI(s) + 3 O2(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
Write an equilibrium expression for each chemical equation involving one or more solid or liquid rea...
Questions





Geography, 10.10.2019 18:20




Geography, 10.10.2019 18:20

Geography, 10.10.2019 18:20

Chemistry, 10.10.2019 18:20






Geography, 10.10.2019 18:20

Geography, 10.10.2019 18:20


![K=\frac{[HCO_3^-][OH^-]}{[CO_3^{2-}]}](/tpl/images/0571/6769/f9db2.png)
![K=[O_2]^3](/tpl/images/0571/6769/a8fdd.png)
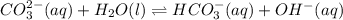
 will be,
will be,