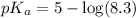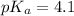The half‑equivalence point of a titration occurs half way to the equivalence point, where half of the analyte has reacted to form its conjugate, and the other half still remains unreacted. If 0.440 moles of a monoprotic weak acid ( K a = 8.3 × 10 − 5 ) is titrated with NaOH , what is the pH of the solution at the half‑equivalence point?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
You know the right answer?
The half‑equivalence point of a titration occurs half way to the equivalence point, where half of th...
Questions

Biology, 26.07.2019 03:00

Biology, 26.07.2019 03:00

Social Studies, 26.07.2019 03:00



Computers and Technology, 26.07.2019 03:00

Mathematics, 26.07.2019 03:00


Computers and Technology, 26.07.2019 03:00

Computers and Technology, 26.07.2019 03:00


Mathematics, 26.07.2019 03:00

Health, 26.07.2019 03:00

Computers and Technology, 26.07.2019 03:00

Social Studies, 26.07.2019 03:00

Biology, 26.07.2019 03:00

History, 26.07.2019 03:00


History, 26.07.2019 03:00

 of weak acid.
of weak acid.

 in this expression, we get:
in this expression, we get: