
Chemistry, 30.03.2020 23:13 slonekaitlyn01
Consider a galvanic cell in which Al 3 is reduced to elemental aluminum and magnesium metal is oxidized to Mg 2 . Write the balanced half-cell reactions that take place at the cathode and at the anode.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
**40** points asapessay questions (10 points possible) clear image of next, create your own scenario. it can be one of your own real experiences or one you make up. use imagery in your writing to give your instructor a the setting and an action taking pace in your writing explain the structure and functions of the skin at work in your scenario. !
Answers: 3

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
You know the right answer?
Consider a galvanic cell in which Al 3 is reduced to elemental aluminum and magnesium metal is oxidi...
Questions




Spanish, 24.11.2020 20:30



Chemistry, 24.11.2020 20:30

Mathematics, 24.11.2020 20:30

Biology, 24.11.2020 20:30

Mathematics, 24.11.2020 20:30

Mathematics, 24.11.2020 20:30


Mathematics, 24.11.2020 20:30

History, 24.11.2020 20:30






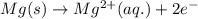 ( × 3)
( × 3)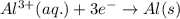 ( × 2)
( × 2)


