
Chemistry, 30.03.2020 23:33 mathman783
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magnitude of k at 75.0°C if Ea = 91.0 kJ/mol? 4.10 × 106 s-1 713 s-1 1.36 × 102 s-1 2.65 × 104 s-1 3.69 × 104 s-1

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1


Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
A particular first-order reaction has a rate constant of 1.35 × 102 s-1 at 25.0°C. What is the magni...
Questions





Social Studies, 28.01.2020 06:31

Biology, 28.01.2020 06:31


Mathematics, 28.01.2020 06:31


History, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31

Advanced Placement (AP), 28.01.2020 06:31

Mathematics, 28.01.2020 06:31

Chemistry, 28.01.2020 06:31

History, 28.01.2020 06:31




Mathematics, 28.01.2020 06:31

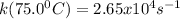
![\frac{k(75.0^0C)}{k(25.0^0C)} =exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]](/tpl/images/0571/8653/57013.png)
![k(75.0^0C)=k(25.0^0C)exp[-\frac{\Delta Ea}{R}(\frac{1}{T_{k(75.0^0C)}}-\frac{1}{T_{k(25.0^0C)}} )]\\\\k(75.0^0C)=1.35x10^2s^{-1}exp[-\frac{91000J/mol}{8.314J/(mol*K)}(\frac{1}{348.15K}-\frac{1}{298.15K} )]\\\\k(75.0^0C)=2.65 x 10^4 s^{-1}](/tpl/images/0571/8653/debb3.png)


