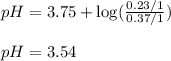Chemistry, 30.03.2020 23:35 joejoefofana
Calculate the pH of a solution prepared by dissolving 0.370 mol of formic acid (HCO2H) and 0.230 mol of sodium formate (NaCO2H) in water sufficient to yield 1.00 L of solution. The Ka of formic acid is

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Given 7.65 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
Answers: 3

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


You know the right answer?
Calculate the pH of a solution prepared by dissolving 0.370 mol of formic acid (HCO2H) and 0.230 mol...
Questions



Computers and Technology, 29.07.2019 20:30



History, 29.07.2019 20:30





Biology, 29.07.2019 20:30

Mathematics, 29.07.2019 20:30



Mathematics, 29.07.2019 20:30




Mathematics, 29.07.2019 20:30

![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0571/8718/e4eea.png)
![pH=pK_a+\log(\frac{[HCOONa]}{[HCOOH]})](/tpl/images/0571/8718/e545d.png)
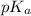 = negative logarithm of acid dissociation constant of formic acid = 3.75
= negative logarithm of acid dissociation constant of formic acid = 3.75![[HCOONa]=\frac{0.230}{1}](/tpl/images/0571/8718/cf36f.png)
![[HCOOH]=\frac{0.370}{1}](/tpl/images/0571/8718/32cdc.png)