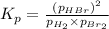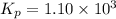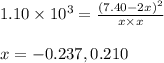Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 23.06.2019 01:30
In what way do investigations build scientific knowledge? the results of investigations lead to questions that cannot be tested. they reflect the opinions and social values of scientists, ensuring valid information. the results of investigations lead to new questions, which lead to new investigations. they are not influenced by the research of earlier scientists, so they are able to address gaps in understanding.i
Answers: 1
You know the right answer?
The equilibrium constant for the following reaction: H2(g) + Br2(g) ↔ 2HBr (g) is 1.10 x 103 at a ce...
Questions


Mathematics, 21.03.2021 22:10

Mathematics, 21.03.2021 22:10

English, 21.03.2021 22:10




Mathematics, 21.03.2021 22:10



Mathematics, 21.03.2021 22:10

Physics, 21.03.2021 22:10

Mathematics, 21.03.2021 22:10

Biology, 21.03.2021 22:10

Mathematics, 21.03.2021 22:10




Mathematics, 21.03.2021 22:10

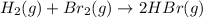
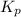 for above equation follows:
for above equation follows: