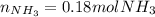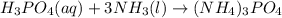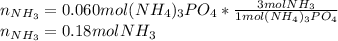Chemistry, 31.03.2020 00:07 caliharris123
Ammonium phosphate is an important ingredient in many solid fertilizers. It can be made by reacting aqueous phosphoric acid with liquid ammonia. Calculate the moles of ammonia needed to produce 0.060 mol of ammonium phosphate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Ammonium phosphate is an important ingredient in many solid fertilizers. It can be made by reacting...
Questions


Mathematics, 01.03.2021 19:10

Chemistry, 01.03.2021 19:10

Computers and Technology, 01.03.2021 19:10




Mathematics, 01.03.2021 19:10


Chemistry, 01.03.2021 19:10





Spanish, 01.03.2021 19:10

English, 01.03.2021 19:10

Mathematics, 01.03.2021 19:10

Mathematics, 01.03.2021 19:10

Mathematics, 01.03.2021 19:10