
Chemistry, 30.03.2020 23:43 tiwaribianca475
If the rate of decrease for the partial pressure of N2H4N2H4 in a closed reaction vessel is 70 torr/htorr/h , what is the rate of change for the partial pressure of NH3NH3 in the same vessel

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
If the rate of decrease for the partial pressure of N2H4N2H4 in a closed reaction vessel is 70 torr/...
Questions





Mathematics, 29.07.2019 18:00

English, 29.07.2019 18:00

Mathematics, 29.07.2019 18:00


History, 29.07.2019 18:00

Mathematics, 29.07.2019 18:00




Health, 29.07.2019 18:00

Mathematics, 29.07.2019 18:00

Mathematics, 29.07.2019 18:00



English, 29.07.2019 18:00

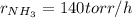
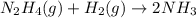
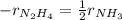
![r_{NH_3}=2*(-r_{N_2H_4})\\r_{NH_3}=2*[-(-70torr/h)]\\r_{NH_3}=140torr/h](/tpl/images/0571/9059/b4613.png)


