
Chemistry, 30.03.2020 23:51 kayyaybruh
For the reaction IO3–(aq) + 5 I–(aq) + 6 H+(aq) → 3 I2(aq) + 3 H2O(l) the rate of disappearance of I–(aq) at a particular time and concentration is 2.4 × 10–3 mol/L · s. What is the rate of appearance of I2(aq)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
For the reaction IO3–(aq) + 5 I–(aq) + 6 H+(aq) → 3 I2(aq) + 3 H2O(l) the rate of disappearance of I...
Questions


Business, 29.11.2021 23:00

Social Studies, 29.11.2021 23:00

Mathematics, 29.11.2021 23:00


Mathematics, 29.11.2021 23:00

Mathematics, 29.11.2021 23:00




Biology, 29.11.2021 23:00






Social Studies, 29.11.2021 23:00

Social Studies, 29.11.2021 23:00


SAT, 29.11.2021 23:00

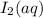 is
is 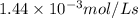
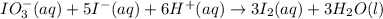
![Rate=-\frac{1d[I^-]}{5dt}=+\frac{d[I_2]}{3dt}](/tpl/images/0571/9488/09ead.png)
![-\frac{d[I^-]}{dt}]](/tpl/images/0571/9488/5d954.png) =
= 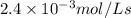
![+\frac{d[I_2]}{dt}=-\frac{3d[I^-]}{5dt}=-\frac{3}{5}\times 2.4\times 10^{-3}mol/Ls=1.44\times 10^{-3}mol/Ls](/tpl/images/0571/9488/1041e.png)


