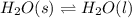Chemistry, 31.03.2020 00:03 cuthbertson157
What was happening to the entropy of the system as the ice melted? It was increasing a lot. It was increasing a little. It was staying about the same. It was decreasing a little. It was decreasing a lot.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
What was happening to the entropy of the system as the ice melted? It was increasing a lot. It was i...
Questions



Business, 20.07.2019 11:30


Mathematics, 20.07.2019 11:30



History, 20.07.2019 11:30


Mathematics, 20.07.2019 11:30

Mathematics, 20.07.2019 11:30


Social Studies, 20.07.2019 11:30

Social Studies, 20.07.2019 11:30

Mathematics, 20.07.2019 11:30




Biology, 20.07.2019 11:30