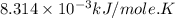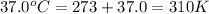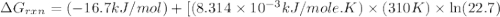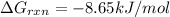Chemistry, 31.03.2020 00:01 samueltaye
Calculate the free energy change if the ratio of the concentrations of the products to the concentrations of the reactants is 22.7 and the temperature is 37.0 ° C ? Δ G ° ' for the reaction is − 16.7 kJ/mol .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Calculate the free energy change if the ratio of the concentrations of the products to the concentra...
Questions

Engineering, 27.08.2021 14:00

English, 27.08.2021 14:00

World Languages, 27.08.2021 14:00



Social Studies, 27.08.2021 14:00




History, 27.08.2021 14:00

History, 27.08.2021 14:00

Mathematics, 27.08.2021 14:00


Mathematics, 27.08.2021 14:00

Chemistry, 27.08.2021 14:00



Biology, 27.08.2021 14:00


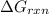 is -8.65 kJ/mol
is -8.65 kJ/mol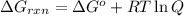 ............(1)
............(1)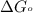 = standard Gibbs free energy = -16.7 kJ/mol
= standard Gibbs free energy = -16.7 kJ/mol