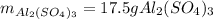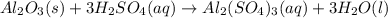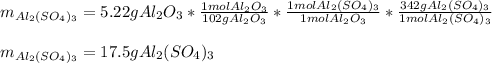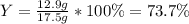Chemistry, 30.03.2020 23:55 guadalupemarlene2001
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The reaction yields 12.9 grams of aluminum sulfate. aluminum oxide (s) + sulfuric acid (aq) aluminum sulfate (aq) + water (l) What is the theoretical yield of aluminum sulfate ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1
You know the right answer?
For the following reaction, 5.22 grams of aluminum oxide are mixed with excess sulfuric acid. The re...
Questions



Mathematics, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31

History, 11.12.2019 07:31

Biology, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31


Mathematics, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31


Mathematics, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31

Biology, 11.12.2019 07:31

Social Studies, 11.12.2019 07:31



Spanish, 11.12.2019 07:31

Mathematics, 11.12.2019 07:31