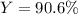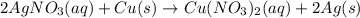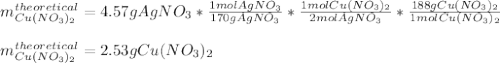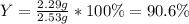For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yields 2.29 gram of copper(II) nitrate What is the percent yield for this reaction?Formula: % yield = (Actual yield/theoretical yield) x 100 2 AgNO3(aq) + Cu(s) à Cu(NO3)2 (aq) + 2 Ag(s)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1

Chemistry, 23.06.2019 11:10
Why would a doctor most likely restrict a patient's contact with other people while the patient receives internal radiation
Answers: 1
You know the right answer?
For the following reaction, 4.57 g of silver nitrate are mixed with excess copper. The reaction yiel...
Questions

English, 24.08.2019 06:30

Social Studies, 24.08.2019 06:30

Chemistry, 24.08.2019 06:30



Mathematics, 24.08.2019 06:30


Mathematics, 24.08.2019 06:30

English, 24.08.2019 06:30

Geography, 24.08.2019 06:30



Computers and Technology, 24.08.2019 06:30



Social Studies, 24.08.2019 06:30

Health, 24.08.2019 06:30

Mathematics, 24.08.2019 06:30

Chemistry, 24.08.2019 06:30

Mathematics, 24.08.2019 06:30