
Chemistry, 31.03.2020 00:14 aubreyfoster
Express the rate of the reaction in terms of the change in concentration of each of the reactants and products.
a. rate=12Δ[HBr]Δt=−Δ[H2]Δt=−Δ[Br2]Δtr ate=12Δ[HBr]Δt=−Δ[H2]Δt=−Δ[Br2]Δt
b. rate=−Δ[HBr]Δt=12Δ[H2]Δt=12Δ[Br2]Δt rate=−Δ[HBr]Δt=12Δ[H2]Δt=12Δ[Br2]Δt
c. rate=−12Δ[HBr]Δt=Δ[H2]Δt=Δ[Br2]Δtra te=−12Δ[HBr]Δt=Δ[H2]Δt=Δ[Br2]Δt
d. rate=Δ[HBr]Δt=−12Δ[H2]Δt=−12Δ[Br2]Δ t

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Express the rate of the reaction in terms of the change in concentration of each of the reactants an...
Questions


History, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01





Biology, 18.10.2020 14:01

History, 18.10.2020 14:01



French, 18.10.2020 14:01


Physics, 18.10.2020 14:01



Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

History, 18.10.2020 14:01

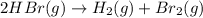
![rate=-\frac{1}{2} \frac{\Delta [HBr]}{\Delta t}=\frac{\Delta [Br_2]}{\Delta t} =\frac{\Delta [H_2]}{\Delta t}](/tpl/images/0572/0368/fc117.png)



