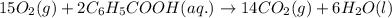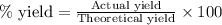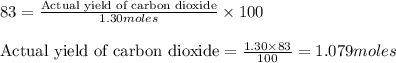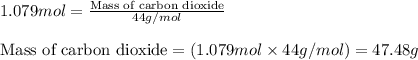Consider the following balanced equation for the following reaction:
15O2(g) + 2C6H5COOH(aq) →...

Chemistry, 31.03.2020 00:12 nicollexo21
Consider the following balanced equation for the following reaction:
15O2(g) + 2C6H5COOH(aq) → 14CO2(g) + 6H2O(l)
Determine the amount of CO2(g) formed in the reaction if the percent yield of CO2(g) is 83.0% and the theoretical yield of CO2(g) is 1.30 moles.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Questions

English, 23.02.2021 23:10

Mathematics, 23.02.2021 23:10


English, 23.02.2021 23:10

Physics, 23.02.2021 23:10



Mathematics, 23.02.2021 23:10




Biology, 23.02.2021 23:10

Mathematics, 23.02.2021 23:10


Physics, 23.02.2021 23:10


Mathematics, 23.02.2021 23:10


Mathematics, 23.02.2021 23:10