
Chemistry, 31.03.2020 00:27 CatsandDogsaredabest
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combustion of 29.5 mg produced 80.1 mg of CO2 and 16.4 mg of H2O. The molar mass of the compound was 162 g/mol. Determine its empirical and molecular formulas.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 7.3 × 106 m/s. show your work. note: h = plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
A compound containing only C, H, and O, was extracted from the bark of the sassafras tree. The combu...
Questions



Mathematics, 05.08.2021 16:20

Physics, 05.08.2021 16:20


Mathematics, 05.08.2021 16:20

Physics, 05.08.2021 16:20




Mathematics, 05.08.2021 16:20




English, 05.08.2021 16:20


Mathematics, 05.08.2021 16:20

English, 05.08.2021 16:30


English, 05.08.2021 16:30

 .
.
 g
g
 g
g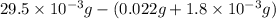
 g
g

 mol
mol

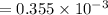 mol
mol



