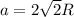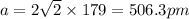Chemistry, 31.03.2020 00:23 tartcandi303
A certain metal M crystallizes in a lattice described by a face-centered cubic (fcc) unit cell. The radius r of M atoms has been measured to be 179.pm. Calculate the lattice constant a of a crystal of M. Be sure your answer has the correct number of significant digits, and be sure it has the correct unit symbol.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2


Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
A certain metal M crystallizes in a lattice described by a face-centered cubic (fcc) unit cell. The...
Questions


Computers and Technology, 30.07.2019 22:50

Geography, 30.07.2019 22:50


Biology, 30.07.2019 22:50




Mathematics, 30.07.2019 22:50


English, 30.07.2019 22:50



History, 30.07.2019 22:50

Mathematics, 30.07.2019 22:50

Physics, 30.07.2019 22:50

Chemistry, 30.07.2019 22:50

Advanced Placement (AP), 30.07.2019 22:50

Spanish, 30.07.2019 22:50