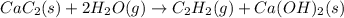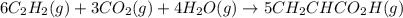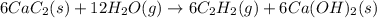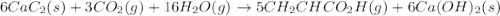Chemistry, 31.03.2020 00:21 isabelperez063
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: (s)(g)(g)(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: (g)(g)(g)(g) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions

Mathematics, 23.11.2020 18:50




Mathematics, 23.11.2020 18:50



Spanish, 23.11.2020 18:50

English, 23.11.2020 18:50




Chemistry, 23.11.2020 18:50

Physics, 23.11.2020 18:50

Mathematics, 23.11.2020 18:50


English, 23.11.2020 18:50