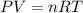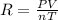Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
If the pressure, volume, and the number of moles of a gas are known, which is needed to calculate th...
Questions

Social Studies, 15.01.2021 01:00


Mathematics, 15.01.2021 01:00


Chemistry, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00

History, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00





Mathematics, 15.01.2021 01:00


English, 15.01.2021 01:00


Physics, 15.01.2021 01:00


Social Studies, 15.01.2021 01:00