
A gas-filled balloon having a volume of 3.60 L at 1.15 atm and 20°C is allowed to rise to the stratosphere (about 30 km above the surface of the Earth), where the temperature and pressure are −50°C and 5.40 × 10−3 atm, respectively. Calculate the final volume of the balloon.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2


Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
A gas-filled balloon having a volume of 3.60 L at 1.15 atm and 20°C is allowed to rise to the strato...
Questions

Mathematics, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31


Mathematics, 11.10.2019 08:31




History, 11.10.2019 08:31


Chemistry, 11.10.2019 08:31

Chemistry, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31

Geography, 11.10.2019 08:31




Mathematics, 11.10.2019 08:31

Mathematics, 11.10.2019 08:31

History, 11.10.2019 08:31

Biology, 11.10.2019 08:31

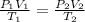
 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas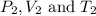 are the final pressure, volume and temperature of the gas
are the final pressure, volume and temperature of the gas![P_1=1.15atm\\V_1=3.60L\\T_1=20^oC=[20+273]K=293K\\P_2=5.40\times 10^{-3}atm=0.00540atm\\V_2=?\\T_2=-50^oC=[-50+273]K=223K](/tpl/images/0572/1398/77ebd.png)



