

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 03:00
What do electromagnetic waves carry? how are they produced through which media can they move? where do they transfer energy? what do they not transfer? what do mechanical waves carry? how are they produced? through which media can they move? where do they transfer energy? what do they not transfer?
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
If the equilibrium constant for the reaction 3I- (aq) + S2O82- (aq) I3- (aq) + 2SO42- (aq) is K, w...
Questions

Mathematics, 29.01.2021 03:50

English, 29.01.2021 03:50


Mathematics, 29.01.2021 03:50

Mathematics, 29.01.2021 03:50

Mathematics, 29.01.2021 03:50


Mathematics, 29.01.2021 03:50


Mathematics, 29.01.2021 03:50

Mathematics, 29.01.2021 03:50

Mathematics, 29.01.2021 03:50

Physics, 29.01.2021 03:50


Mathematics, 29.01.2021 03:50


History, 29.01.2021 03:50


Chemistry, 29.01.2021 03:50

History, 29.01.2021 03:50

![K'=\sqrt[3]{\frac{1}{K}}](/tpl/images/0572/2471/9553b.png)
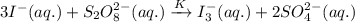 ......(1)
......(1)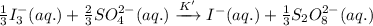 .........(2)
.........(2)

