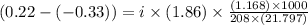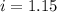Chemistry, 31.03.2020 01:05 raishagibson
Assume that you were assigned BaCl2 in lab. The water in your test tube weighed 21.797 g. Following the procedure in the lab manual, you determined that freezing point of water is 0.02oC. You weighed out 1.168 g of salt and added it to the original test tube, then determined that the freezing point was -0.33oC. Based on these experimental parameters, calculate the van't Hoff factor for BaCl2.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2


Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
Assume that you were assigned BaCl2 in lab. The water in your test tube weighed 21.797 g. Following...
Questions

Mathematics, 03.03.2021 01:00



Mathematics, 03.03.2021 01:00




Spanish, 03.03.2021 01:00






Mathematics, 03.03.2021 01:00

English, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00




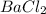 is 1.15
is 1.15
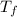 = freezing point of solution =
= freezing point of solution = 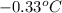
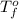 = freezing point of water =
= freezing point of water = 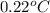
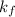 = freezing point constant of water =
= freezing point constant of water = 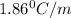
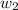 = mass of solute = 1.168 g
= mass of solute = 1.168 g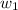 = mass of solvent (water) = 21.797 g
= mass of solvent (water) = 21.797 g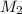 = molar mass of solute = 208 g/mol
= molar mass of solute = 208 g/mol