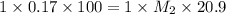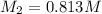Chemistry, 31.03.2020 01:05 bjpvrpow74wq
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3.56 grams of potassium hydrogen phthalate (KHP) into 100 mL of H2O. You then titrate the KHP with your sodium hydroxide solution and reach the endpoint after adding 20.9 mL of NaOH. What is the molarity of your sodium hydroxide solution? (Molecular Mass of KHP = 204.22 g/mol)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
To standardize a solution of NaOH before using it in a titration of an unknown acid, you dissolve 3....
Questions

Mathematics, 05.01.2021 23:50

Chemistry, 05.01.2021 23:50


Mathematics, 05.01.2021 23:50

Mathematics, 05.01.2021 23:50



World Languages, 05.01.2021 23:50

History, 05.01.2021 23:50


Mathematics, 05.01.2021 23:50

Social Studies, 05.01.2021 23:50

Mathematics, 05.01.2021 23:50

Chemistry, 05.01.2021 23:50





Mathematics, 05.01.2021 23:50

Mathematics, 05.01.2021 23:50

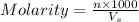
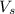 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml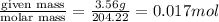
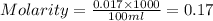
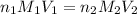
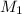 = molarity of KHP solution = 0.17 M
= molarity of KHP solution = 0.17 M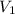 = volume of KHP solution = 100 ml
= volume of KHP solution = 100 ml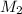 = molarity of NaOH solution = ?
= molarity of NaOH solution = ?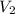 = volume of NaOH solution = 20.9 ml
= volume of NaOH solution = 20.9 ml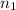 = valency of KHP = 1
= valency of KHP = 1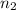 = valency of NaOH = 1
= valency of NaOH = 1