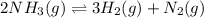Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Consider the following system at equilibrium at 723 K: 2 NH3 (g) 26.6 kcal N2 (g) 3 H2 (g) Indicate...
Questions


Social Studies, 31.07.2019 04:00

Mathematics, 31.07.2019 04:00

Mathematics, 31.07.2019 04:00


Biology, 31.07.2019 04:00





Mathematics, 31.07.2019 04:00

English, 31.07.2019 04:00




Mathematics, 31.07.2019 04:00


Mathematics, 31.07.2019 04:00

Arts, 31.07.2019 04:00

English, 31.07.2019 04:00