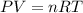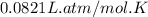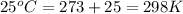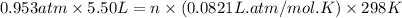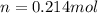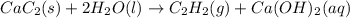Chemistry, 31.03.2020 00:57 MajentaSnow66
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2(s) + 2H2O(l)C2H2(g) + Ca(OH)2(aq) The product gas, C2H2, is collected over water at a temperature of 25 °C and a pressure of 748 mm Hg. If the wet C2H2 gas formed occupies a volume of 5.50 L, the number of moles of CaC2 reacted was mol. The vapor pressure of water is 23.8 mm Hg at 25 °C.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1
You know the right answer?
Calcium carbide reacts with water to produce acetylene gas according to the following equation: CaC2...
Questions

Mathematics, 22.08.2021 20:10

Social Studies, 22.08.2021 20:10

Mathematics, 22.08.2021 20:10



Biology, 22.08.2021 20:10



Mathematics, 22.08.2021 20:20


Mathematics, 22.08.2021 20:20


Biology, 22.08.2021 20:20

History, 22.08.2021 20:20

Mathematics, 22.08.2021 20:20

Mathematics, 22.08.2021 20:20


Business, 22.08.2021 20:20

Mathematics, 22.08.2021 20:20

Mathematics, 22.08.2021 20:20

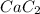 reacted was, 0.214 moles.
reacted was, 0.214 moles.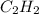 gas.
gas.